Austrian Researchers Develop New Formulation of Diacerein to Treat EBS
Written by |
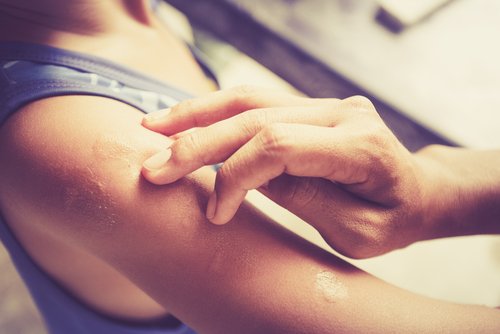
Diacerein, which is a commercially available medicine used to treat osteoarthritis, also may hold therapeutic potential to treat blistering in patients with epidermolysis bullosa simplex type Dowling-Meara (EBS-DM).
Initially discovered by a team of researchers at the EB House Austria, University Clinic for Dermatology at the Paracelsus Medical University (PMU), the new treatment is being developed by Castle Creek Pharma, and is under Phase 2/3 clinical trial evaluation.
EBS-DM is one of the most severe forms of epidermolysis bullosa. Caused by genetic mutations in keratin 5 or keratin 14, patients develop blisters, experience skin erosions, and formation of mucous membranes when exposed to minor trauma. Characterized by pain and pruritus, this illness can affect a person’s quality of life severely.
“In the other forms of EB, the blisters are a direct consequence of the weakened skin … But in the simplex variant that we are targeting this weakness is less-pronounced. While the protein is still present, it mutates and clumps within the cells. This triggers inflammatory reactions” Johann Bauer, head of the University Clinic for Dermatology at PMU and medical director of EB House Austria, said in a press release on the official blog of the Austrian Science Fund (FWF). FWF supported this project.
In preclinical studies led by Bauer, researchers found that a signaling protein called interleukin-1β, or IL-1β, was present in higher levels in skin cells of EBS-DM that are responsible for producing keratin (keratinocytes). This finding caught the researchers’ attention not only because IL-1β is an important pro-inflammatory protein, but also because it is known to be involved in wound-healing processes and keratinocytes activation.
The team wondered if blocking this signaling protein could have a positive impact on EBS-DM patients’ outcome. To test this hypothesis, they used the drug diacerein, which is an inhibitor of IL-1β activity and is approved for the treatment of osteoarthritis (commercially available with the trade name Verboril).
This drug is commonly marketed as tablets, but for its use in EBS-DM patients, a new formulation was needed that could be applied directly to the skin. Researchers then developed an ointment with 1 percent of diacerein that they tested in a pilot study in five patients with EBS-DM.
“We were astonished by the success” said Bauer. “Within two weeks we observed a reduction of 80% of the blisters. And to our surprise – we still don’t really know why – we brought about a very lasting effect. The blisters did not reappear even without treatment.”
The results of the pilot study were published in the Orphanet Journal of Rare Diseases, in an article titled “Topical diacerein for epidermolysis bullosa: a randomized controlled pilot study.”
Supported by the positive clinical results, the new topical diacerein formulation received orphan drug designation status by the European Medicines Agency (EMA).
Currently the investigative treatment is being further developed by Castle Creek Pharma, a U.S. start-up pharmaceutical company, under a licensing agreement.
The diacerein 1% ointment topical formulation, also named CCP-020, is being tested in a Phase 2/3 clinical trial (NCT03154333) in patients with EBS. This DELIVERS study is expected to enroll about 80 participants across 18 clinical sites in the U.S., Europe, and Israel. Currently recruiting patients, the study is going to test the safety and effectiveness of CCP-020 in comparison to a placebo.
“We hope that it all goes well and that we receive approval soon, first in the USA and then in Europe,” Bauer said.





