Wound-specific Bacteria May Be Targets for DEB Treatment
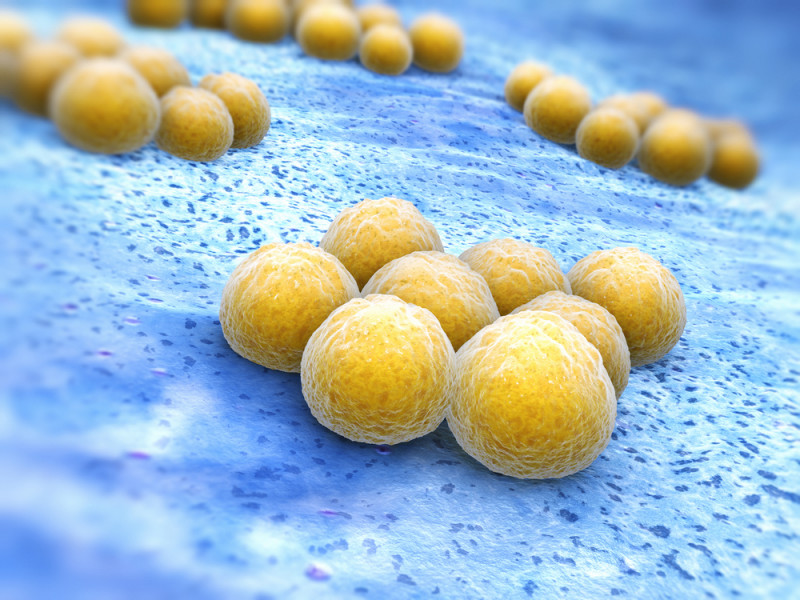
A distinct set of bacteria colonizes the skin in and around wounds in people with dystrophic epidermolysis bullosa (DEB), according to a new study.
The finding suggests that these patients may benefit from therapies that specifically target those bacteria and that encourage the growth of non wound-associated bacteria instead.
The study, “Evidence for cutaneous dysbiosis in dystrophic epidermolysis bullosa,” was published in the journal Clinical and Experimental Dermatology.
In addition to acting as a barrier to the surrounding environment, the skin houses a wide variety of microbes — collectively called the “skin microbiome” — that form an integral part of normal skin homeostasis (equilibrium or a relatively stable balance). Disruptions to the balance of microbial communities is known as “dysbiosis.”
Dysbiosis has been implicated in a number of skin disorders, including DEB. However, according to the research team, only one study examined the DEB skin microbiome using advanced genetic sequencing, which provides more detailed information on the microbiome than traditional studies using bacterial cultures.
Now, the team, led by scientists from the Tel Aviv Sourasky Medical Center, in Israel, compared skin microbiomes of eight DEB patients and nine age- and sex-matched healthy controls.
The researchers collected skin samples from untreated ulcers with no evidence of secondary infections, the skin bordering the wounds (perilesional skin), and unwounded (normal) skin. For comparison, they sampled the forearm skin of healthy participants.
The microbial composition found on the patients’ skin differed from those of controls, with fewer species overall found on the skin of patients. Perilesional skin and wounds of patients appeared have the least diversity.
Although home to fewer species, their skin microbiomes showed greater proportions of bacterial Staphylococci species, with Staphylococcus epidermidis being the most prevalent. This trend was particularly observed in wounds and perilesional skin, compared with their normal skin.
Staphylococcus epidermidis normally inhabits human skin as a healthy component of the microbiome, or what is known as a “commensal bacteria.” These bacteria may turn pathogenic when the barrier of the skin fails, or in cases of immunosuppression, the investigators noted. This might explain the higher abundance of these bacteria in DEB wounds, they stated.
This type of bacteria also has the ability to form biofilms, a type of sticky plaque that can often resist antibacterial treatments.
“The increased abundance of Staphylococcus epidermidis in DEB patients emphasizes the potential therapeutic effect of antimicrobial peptides harboring the ability to eradicate Staphylococcus epidermidis biofilms,” the researchers wrote, especially as “bacterial colonization of chronic EB wounds has been suggested to be responsible for delayed healing and scarring.”
Despite the study’s small size, “patients demonstrated similar individual profiles,” the team noted, suggesting that the results might be broadly representative of this patient population.
Overall, the “results provide evidence for significant dysbiosis across different stages of DEB wound,” the researchers concluded. They suggested that therapies targeting wound-specific bacteria or encouraging the growth of non wound-associated bacteria “may potentially facilitate wound healing in DEB patients.”
“The benefits of such therapeutic approaches should be ascertained,” the team added.