Healiva and C4U to team up on new gene editing therapy for EB
Partners to use C4U's CRISPR-Cas3 technology to correct gene mutations
Written by |
Healiva and C4U will collaborate to develop a next-generation gene editing therapy — focusing on new technology to correct gene mutations — for epidermolysis bullosa (EB) patients, who are sometimes known as butterfly children for their fragile skin.
Combining C4U’s expertise in gene editing technology and Healiva’s experience with cell-based treatments for wound healing, the companies will work together through a staged development process.
Their goal is to accelerate the development of an investigational therapy toward clinical testing.
“The collaboration aims to provide answers and hope to the individuals affected by EB, a devastating skin condition that renders the skin of the affected children as fragile as butterfly wings,” Valerio M. Ferrari, Healiva’s co-founder and chairman of the Bioseutica Group said in a joint press release from the new partners. Bioseutica is one of the companies that launched Healiva.
Gene replacement therapy now approved for dystrophic EB
EB encompasses a group of rare skin disorders characterized by very fragile skin that easily blisters and tears. In most cases, the disease is caused by mutations in genes that encode production of proteins important for the skin’s integrity.
Because most types of EB have known genetic causes, gene therapies to replace the faulty gene or correct the disease-causing mutation are of significant interest.
A topical gene replacement therapy called Vyjuvek (beremagene geperpavec) was approved by the U.S. Food and Drug Administration earlier this year for treating wounds in people with dystrophic EB, commonly known as DEB. It provides patients’ skin cells with a working version of the COL7A1 gene that’s implicated in that form of the disease.
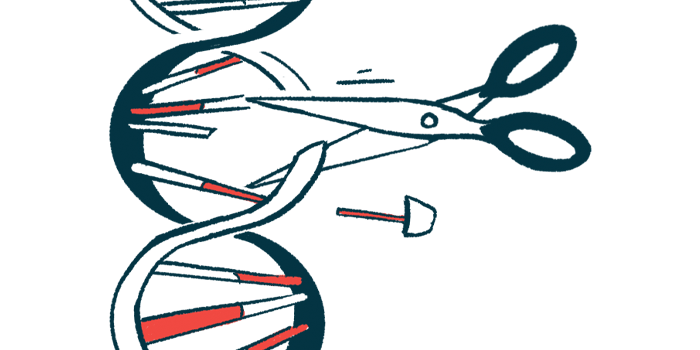
A gene editing therapy uses somewhat different technology and aims to correct the mutation in a person’s gene rather than replace the gene altogether. As such, gene editing would deliver to cells the material needed to make necessary changes.
A common gene editing tool is called the CRISPR/Cas system, which is derived from bacteria’s natural defense systems against viruses. It contains two key components — a Cas enzyme and a guide molecule.
The Cas enzyme is responsible for making the cuts to the DNA that enable it to be edited, while the guide molecule binds to a specific part of the gene and tells Cas exactly where to make its cuts. That cut allows genetic material to be deleted or inserted in that location.
There are different types of Cas enzymes, which work to cut the DNA in slightly different ways. The standard one is called Cas9, but C4U has been working on using Cas3 for the treatment of genetic diseases.
Skin diseases, especially EB, are relevant targets for the CRISPR-Cas3 platform.
Akimitsu Hirai, C4U’s president and CEO, called the company’s proprietary CRISPR-Cas3 platform “a new generation of gene editing technology.”
The company, based in Japan, believes this enzyme will have a few advantages, including avoiding off-target DNA cuts that can compromise the safety of gene-editing tools. It’s also better than Cas9 for cutting out larger stretches of DNA.
“C4U’s focus is to develop alternative and complete cures to genetic diseases,” Hirai said, adding that “skin diseases, especially EB, are relevant targets for the CRISPR-Cas3 platform.”
The Switzerland-based Healiva will bring to the table its expertise in developing cell therapies for the treatment of wound care and skin diseases.
“This collaboration integrates gene therapy prospects with our comprehensive cell therapy portfolio,” said Priyanka Dutta Passecker, PhD, Healiva co-founder and CEO.